There are many methods of reaching understanding - all begin with the use of one, two, three, four or all five of our senses.
What we do with the input, the information, the raw "data" we gather, ... is the
How we process for understanding.
Skill + Knowledge = Understanding
How we process for understanding.
Skill + Knowledge = Understanding
The precision of SKILL we use to process the information or KNOWLEDGE,
becomes our UNDERSTANDING
becomes our UNDERSTANDING
There are so many ways , so many paths to understanding and discovery..
....inductive reasoning, deductive reasoning, (favorites of a famous investigator )...logical organization, scientific methodology etc.
One very powerful way is; similarity comparison, metaphor or analogy.
I am proud to share one from one of my students.
For example;
The Periodic table can appear daunting , complex and even a bit scary...

Here is a way one of our own "Sherlock Holmes" clues us into an understanding of the periodic table.
Sherlock;
If anyone needs help with the periodic table? If so read on. ( think about what we're studying in Mr. Nevins class 4.3 especially)

Helium, Neon,Argon, Krypton, Xenon, Radon, and Ununoctium, they're the plantation owners (Noble gases) and self-sufficient.
-test your knowledge of the Noble elements -
Independent small farmers (Metals) aren't self-sufficient and need help to be whole.
Nonmetals are immigrants that aren't considered high in society, but are still regarded.
Slaves (transitional - the are really odd. find out why!) aren't considered fully human (closer to animals), so they don't fit in with anyone.
Sherlock/yellow
P.S. Hope this helps and I couldn't figure out who the Lanthanide and Actinide Series could be ???
...discovery is fascinating...
And now another investigator continues with metaphor and analogy... how cool is this ?!?
**ALSO...
...and Like how the enslaved natives and the Africans weren't very different from one another (except how the natives escaped slavery and the Africans did not)Alkali metals and Alkali earth metals aren't very different from one another as well...
The main difference between the two is their atomic configuration (an alkali earth metal has two electrons in it's outer-most energy level- valence , and alkali metals only have a single electron, this means that they are both highly reactive).
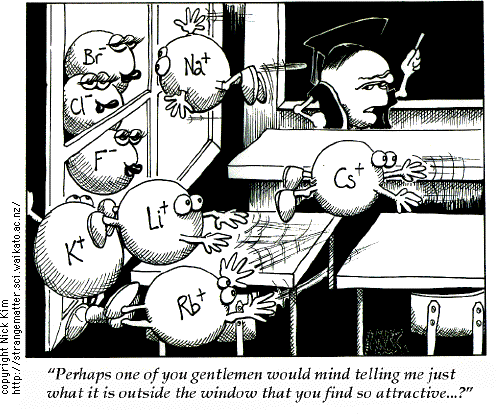
Alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium) make up Group 1 on the
 Periodic Table.
Periodic Table.Alkali earth metals (beryllium, magnesium, calcium,strontium, barium, radium)make up Group 2 on the Periodic Table.
****To always remember alkali metals & alkali earth metals' places on the Periodic Table is that how the Native Americans were first to escape enslavement then the Africans (alkali metals [the "Natives"] are first to the very left of the Periodic table, then the alkali earth metals [the "Africans"]come right after them...***
-Kaitlyn, Yellow
the Analysis continues:

**ALSO...
ReplyDeleteLike how the enslaved natives and the Africans weren't very different from one another (except how the natives escaped slavery and the Africans did not)Alkali metals and Alkali earth metals aren't very different from one another as well...
The main difference between the two is their atomic configuration (an alkali earth metal has two electrons in it's outer-most energy level,and alkali metals only have a single electron, this means that they are both highly reactive). Alkali metals(lithium, sodium,potassium,rubidium, cesium,and francium) make up Group 1 on the Periodic Table. Alkali earth metals(beryllium, magnesium, calcium,strontium, barium, radium)make up group 2 on the Periodic Table.
****To always remember alkali metals & alkali earth metals' places on the Periodic Table is that how the Native Americans were first to escape enslavement then the Africans (alkali metals [the "Natives"] are first to the very left of the Periodic table, then the alkali earth metals [the "Africans"]come right after them...***
-Kaitlyn, Yellow
Wow! Sheelock (Savannah)You are Brilliant. Thank you!
ReplyDeleteMr. V, I have a question about Primary Question number 4. Describe the relation between energy and matter change of state. I don't understand what it is asking. Would you please explain this to me, either in a response to this or in class on monday?
Thank you
Savanah Yellow
Thank you also for the description of how to play football in class on friday. I actually understand it now. I don't particularly care for watching it, but niether of my parents have ever explained it very well.
ReplyDeleteSavanah Yellow
Would you also explain Net Force to me? I understand that it is a combination of all the forces acting on an object but I don't understand the formula. Fnet = F1 + F2 +
ReplyDelete{ F1, F2, … }
Savanah Yellow
Mr. V, I found a website that explained atomic orbitals to me.
ReplyDeletehttp://www.chemguide.co.uk/atoms/properties/atomorbs.html
Savanah
ReplyDeleteThink about Primary Question 4 this way... What role(s) do energy and matter play in changes of state?
think of examples...i.e.
When water changes state from a solid to a liquid what are(describe)the matter changes? (think molecules) What are (describe)the energy changes? (Think heat)
and remember ..
Increase, Decrease, stay the same, that's the name of the science game.
Kaitlyn - thats a big 20!!!!
ReplyDeleteIf a force of 5 newtons is with an upward vector is met with a force of 5 newtons with a down vector ... What is a the Net force>
ReplyDelete(We will be reviewing this in detail.. soon.
Good Q... put two(2) in the box.
Great website... whoever you are !
ReplyDeleteSavanah, glad I could help with the football metaphor / analogy ....
ReplyDeleteJust remember, grown ups will do weird things, go sort of crazy, and spend lots of money when it comes to a "dead pig"
Mr. Voskuil are progress logs due tom. Cuz u weren't here today to grade? But I also hope you feel better :)
ReplyDeleteSabrina, yellow
WednesdaY
ReplyDeleteMr. V it was me who found the website, I just forgot to put my name.
ReplyDeleteSavanah Yellow
Ya know, I kinda figured.
ReplyDeleteSherlock just wants to say:
ReplyDeleteAWESOME JOB KAITLYN!!!
QUESTION 1)Can I use the Atomic Dating Theory?
( I find it easy to understand, and if others want to use the Atomic Dating Theory, I give my permission)
Question 2) Whats the formula for calculating neutrons? DO we need to know the formula?
Sherlock/Yellow
By the way if anyone is bored and wants to challenge me to write a story about a concept, I'll take it! :)
Neutrons = Atomic Mass - # of protons
ReplyDeleteOh just saying I might not be able to answer tonight so don't nag if I don't answer right away please. Also if anyone wants a story about things we learned in 6th and 7th grade to review for FCAT, I can try that too. Just give me a topic nd i will try it out.
ReplyDeleteSherlock/yellow
Mr V. I'm being honest here. I don't have any paper or notes in front of me right now and thats the truth. I'm going to tell you all 18 element we should know already in order with their symbol (for my test preparation):
ReplyDelete1. Hydrogen (H) Non-Metal
2. Helium (He) Inert Element
3. Lithium (Li) Alkali Metal
4. Beryllium (Br) Alkali Earth Metal
5. Boron (B) Non-metal
6. Carbon (C) Non-metal
7. Nitrogen (N) Non-metal
8. Oxygen (O) Non-metal
9. Fluorine (F) Halogen
10. Neon (Ne) Inert Element
11. Sodium (Na) Alkali Metal
12. Magnesium (Mg) Alkali Earth Metal
13. Aluminum (Al) Other Metal
14. Silicon (Si) Non-metal
15. Posphorous (P) Non-metal
16. Sulfur (S) Non-metal
17. Chlorine (Cl) Halogen
18. Argon (Ar) Inert Element
Inert Element is also a noble gas.
*I'm ready for the quiz bring it on.
Tyler/Green
I like the Sir Arthur Conan Doyle allusion. (even though I didn't have to know these)
ReplyDeleteThanks!
-Levi
green
Mr V!!!
ReplyDeleteGo on youtube and type in the "element song" by Tom lehrer!
Kaitlyn Yellow
Thanks Levi. ( everyone should know at least the first 18) What you missed right before you joined us is the Elements for Life project. Each student (or pair) researched one of the 27/28 elements that are essential for life; what, how and why they are. Some great presentations and important understandings came out of that lesson.
ReplyDeleteLevi, That's two(2) in the Bonus box.
ReplyDeleteI luv the element song! Spread the word.
ReplyDeletethat's 2 for finding it ...how many do you think I should make available to any one who can sing it?
10 for singing!! It's difficult...especially since the song doesn't list the elements in order
ReplyDelete-Kaitlyn yellow
Okay... 11
ReplyDeleteIm really confused on the pg. 161 assignment and so can you please explain it to me ?
ReplyDeleteThank you !
Lindsey,GREEN
You need to pair up positive and negative ions in proper ratios to that the compound is stable (neutral)
ReplyDeleteA 1+ ion paired with a 1- ion would be stable(neutral)
A 2+ ion paired with two 1- ions would be stable(neutral) use a subscript of "2" after the 1- ion to show there are two of them.
Don't make it more difficult than it is.
Let me know if this helps.
Answers to page 163 Assessment:
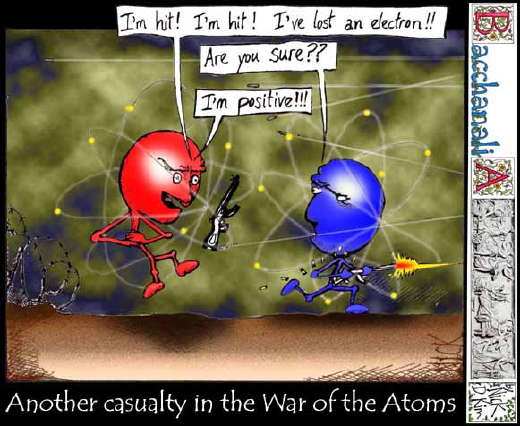
ReplyDelete1. a. Atoms can lose or gain Electrons
b. A sodium ion (Na+) forms when a sodium atom loses one electron and becomes positively charged. A chloride ion (Cl-) forms when a chlorine atom gains one electron and becomes negatively charged.
c. An ionic bond holds the ions together. The sodium atom has a 1+ charge and the chlorine atom has a 1- charge.
2. a. The ratio of positive ions to negative ions
b. Sodium sulfide consists of two (2) sodium ions to every one (1) sulfide ion.
c. The formula is CaCl2, because two chloride ions (Cl-) are needed to balance one calcium ion (Ca2+).
3. a. Ionic compounds are hard, brittle crystals that have high melting points and can conduct electricity when melted or in solution.
b. Ionic bonds are strong due to the attraction between oppositely charged ions. This makes crystals of ionic compounds hard and brittle and causes the compounds to have high melting points. Because the ions are charged, they can conduct electricity when the compound is melted or dissolved.